Abstract
Introduction Allogeneic hematopoietic cell transplantation (HCT) is the only potentially curative treatment for many high-risk hematologic malignancies. Myeloablative conditioning (MAC) is currently the standard of care for young and fit patients; however, graft-versus host disease (GVHD) continues to be a major limitation to the success of HCT, increasing post-transplant morbidity and mortality. An ideal HCT is one that combines strategies to reduce the incidence and severity of GVHD, without compromising graft versus-tumor effect. We hypothesized that the GVHD prophylaxis regimen consisting of post-transplant cyclophosphamide (PTCy), tacrolimus (Tac) and mycophenolate mofetil (MMF) would reduce the incidence of chronic GVHD in patients receiving a standard MAC HCT without an increase in risk of malignant relapse.
Methods This is an analysis of a phase II study at the University of Minnesota using a MAC regimen of either: 1. total body irradiation (TBI, total dose 1320 cGy administered twice a day from days -4 to -1) or 2. Busulfan 3.2mg/kg daily (cumulative AUC 19,000 - 21,000 μmol/min/L) plus fludarabine 160mg/m2 days -5 to -2 for patients unable to receive further radiation, followed by a GVHD prophylaxis regimen of PTCy (50mg/kg days +3 and +4), Tac and MMF (beginning day +5). The primary endpoint is cumulative incidence of chronic GVHD requiring systemic immunosuppressive treatment at 1 year post-transplant.
Eligibility included: age ≤ 60 years, malignant or non-malignant diagnosis, matched related (MRD) or unrelated (MUD) donor with either a bone marrow (BM) or filgrastim-mobilized peripheral blood (PB) graft. We compared those results to our previous MAC protocol which utilized a regimen of either cyclophosphamide (60mg/kg days -6 and -5)/TBI (1320 cGy administered twice a day from days -4 to -1) or busulfan (0.8 mg/kg/dose IV in 4 daily doses days -9 to -6 [total 12.8 mg/kg])/ cyclophosphamide (50 mg/kg days -5 to -2) and GVHD prophylaxis consisted of cyclosporine and methotrexate.
Results Through June 2022, we treated 125 patients with a median follow up of 472 days post-transplant. Of those, 48% were female and 22 (17.6%) were ≤ 18 years old with median age at HCT of 38 years (range, 1-59 yrs; Interquartile range [IQR], 22-50 yrs). Donor source was 8/8 MRD in 69 patients (55.2%), 8/8 MUD in 44 (35.2%), and 7/8 MUD in 12 (9.6%). Graft source was bone marrow in 43 patients (34.4%) and PB in 82 (65.6%). Preparative regimen was TBI in 89.6% of patients. 124 of 125 patients achieved primary neutrophil engraftment by 42 days, median 16 days (range, 13-30). 119 of 125 achieved platelet engraftment by 6 months, median 25.5 days (range, 14-98). 91 patients (72.8%) required total parenteral nutrition (TPN) for oral mucositis and regimen-related toxicities during their initial transplant admission.
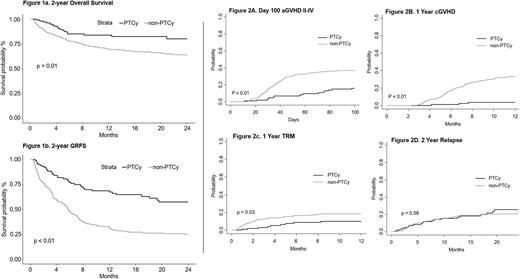
Cumulative incidence of Grade II-IV acute GVHD by day +100 was 16% (95% confidence interval (CI): 9-23%); Grade III-IV acute GVHD was 4% (CI: 0-8%). No patients experienced grade IV GVHD and there were no deaths due to GVHD. At 1 year, only 3 of 105 patients developed chronic GVHD requiring immune suppression, for a cumulative incidence of 4% (CI: 0-8%). Two-year cumulative incidence of relapse was 25% (CI: 15-36%). Two-year overall survival was 80% (CI: 69-87%) and two-year GVHD-free relapse free survival (GRFS) was 57% (CI: 45-67%). Kaplan-Meier curves comparing overall survival and GRFS between this cohort and our previous MAC protocol without PTCy are shown in Figure 1. Figure 2 shows the comparison between these two consecutive cohorts in incidence of acute and chronic GVHD, transplant-related mortality (TRM) and relapse.
Discussion Myeloablative HCT with a TBI or Busulfan/Fludarabine-based preparative regimen, followed by a GVHD prophylaxis regimen of PTCy, Tac, and MMF results in extremely low incidence of chronic GVHD, the primary objective of this clinical trial. Importantly, this regimen is feasible and effective for pediatric and adult patients. Further improvement in outcomes could potentially be achieved by incorporating post-transplant relapse mitigating strategies as well as supportive care measures to decrease regimen-related toxicities.
Disclosures
Gupta:Blue Rock Therapeutics: Membership on an entity's Board of Directors or advisory committees; Vertex Pharmaceuticals: Consultancy. Maakaron:Gilead: Research Funding; CRISPR Therapeutics: Research Funding; Precision BioSciences: Research Funding; Scripps: Research Funding; Fate Therapeutics: Research Funding; ADC Therapeutics: Research Funding. Betts:Brian Betts: Patents & Royalties: WO2019165156; CTI Biopharma: Honoraria; Incyte: Honoraria. Bachanova:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; FATA Therapeutics: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Citius Pharma: Research Funding; Karyopharma: Consultancy; Gamida Cell: Membership on an entity's Board of Directors or advisory committees, Research Funding. Miller, MD:ONK Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Vycellix: Consultancy, Current holder of stock options in a privately-held company; GT Biopharma: Consultancy, Current holder of stock options in a privately-held company, Research Funding; Fate Therapeutics: Consultancy, Current holder of stock options in a privately-held company, Research Funding. Wagner:ASC Therapeutics: Consultancy; Bluebird Bio: Consultancy; Vertex Pharmaceuticals: Consultancy; Magenta Therapeutics: Consultancy; Rocket Pharmaceuticals, Inc.: Consultancy, Current equity holder in publicly-traded company. Vercellotti:Mitobridge-Astellas: Research Funding; Omeros: Research Funding; CSL-Behring: Research Funding. Weisdorf:FATE Therapeutics: Other: Research Support; Incyte: Other: Research support. Holtan:Vitrac Therapeutics: Research Funding; Incyte: Research Funding; Ossium: Consultancy; Generon: Consultancy; CSL Behring: Other: Clinical trial adjudication.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal